Prostate Cancer Organoids Open Path to Precision Oncology
Nov 16, 2021 — Atlanta, GA
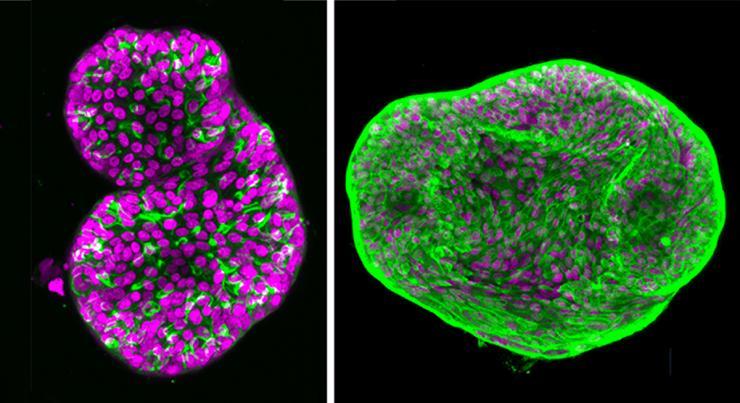
Two organoids: The Matrigel-based model of prostate cancer is at left; the new, improved synthetic hydrogel model is at right.
A multi-institutional team of investigators led by bioengineer Ankur Singh has developed research tools that shed new light on a virtually untreatable form of prostate cancer, opening a pathway that may lead to novel therapeutics and a glimmer of hope for patients.
Androgen receptor pathway inhibitors can prolong survival for patients with advanced prostate cancer. But about 20 perecent of patients develop more advanced-stage neuroendocrine prostate cancer in response to this type of hormone therapy, and so far, researchers haven’t had effective ways to study that progression.
“These patients lose their dependency on hormone-driven processes, and conventional treatments don’t work for them,” said Singh, associate professor in both the Wallace H. Coulter Department of Biomedical Engineering at Emory University and Georgia Tech and the George W. Woodruff School of Mechanical Engineering at Tech.
“There are no targeted therapies, so there is a clear clinical need,” he added. “But a major challenge is, we don’t fully understand what these tumors entail, the kind of tumor microenvironment it has, or the factors that induce resistance to therapeutics. There are no models to effectively study this cancer.”
“To begin to answer those questions, Singh and his team developed a prostate cancer organoid that can help them model the patient-specific microenvironment. It could offer an important step forward in precision medicine, and they described it in the November issue of the journal Advanced Materials.
Organoids are tiny, three-dimensional tissue cultures grown from a patient’s cells. They can be engineered to replicate different organs of the human body, or to model diseases. Produced entirely in vitro, organoids are valuable tools for researchers, who can explore targeted treatments in authentic human micro-anatomies without harming a patient.
Scientists grow organoids in a gel that acts as the extracellular matrix — the protein-rich molecular network that surrounds and supports cells in the body, helping them attach to and communicate with one another and playing a key role in multiple cell functions.
Singh’s collaborators on this study had previously developed Matrigel organoid models of neuroendocrine prostate cancer — that is, they grew cells in Matrigel, a naturally derived solution from mouse tumor cells. Using these organoids, the researchers had discovered a new therapeutic target called EZH2, a histone-modifying protein that promotes tumor growth. Using an EZH2 inhibitor, they were able to slow tumor growth.
“EZH2 inhibitors may require high doses, and we are just beginning to understand factors that control EZH2 activity. And, in some patients, EZH2 inhibitors may not eliminate the tumor in its entirety,” Singh said.
Reasoning that the EZH2 inhibitor would reach full potential in the right kind of tumor microenvironment, something they could design — i.e., not Matrigel — they analyzed 111 patient biopsies using a multi-omics approach and microscopy techniques to thoroughly profile these aggressive tumors.
Their findings helped them design and develop a synthetic, Maleimide-polyethyleneglycol-based hydrogel that accurately mimics the extracellular matrix of a patient-specific tumor. Using these organoids, the researchers were able to study the impact of the matrix on tumor development — particularly the changes associated with transforming a treatable prostate cancer tumor into an untreatable one.
With the new organoids, they discovered that extracellular matrix regulates EZH2 activity and the efficacy of EZH2 inhibitors, a previously less understood phenomenon. They also discovered a potential new therapeutic target, a molecule called DRD2. Currently, DRD2 inhibitors are being tested in clinical trials for gliomas, but they have never been tested in neuroendocrine prostate tumors.
Singh’s team found that certain extracellular matrices found in patients could render neuroendocrine tumors resistant to DRD2 inhibitors, but the resistance could be overcome with a combination therapy: first, an EZH2 inhibitor to reprogram the cells and make them more susceptible to DRD2 inhibition.
“As a single-agent targeted therapeutic, DRD2 is very exciting,” said Singh, whose collaborators included co-lead investigator Oliver Elemento, director of the Englander Institute for Precision Medicine at Weill Cornell Medicine, the biomedical research unit and medical school of Cornell University. The lead author was Matthew Mosquera, a former Ph.D. student in Singh’s lab.
Singh believes this work could evolve into a new standard of precision medicine.
“Not every patient’s tumor microenvironment is the same,” Singh said. “We could take a biopsy sample, profile the patient’s microenvironment, take that specific information and create an organoid model that you can treat with drugs and develop a personalized treatment regime. Tailoring this towards precision oncology would be pretty huge for us. That was original idea. That is the ultimate goal.”
CITATION: Matthew Mosquera, etc. “Extracellular Matrix in Synthetic Hydrogel-Based Prostate Cancer Organoids Regulate Therapeutic Response to EZH2 and DRD2 Inhibitors.” Advanced Materials. https://doi.org/10.1002/adma.202100096
Writer: Jerry Grillo




