A New Carbon-Negative Method to Produce Essential Amino Acids
Nov 21, 2024 —

Glycine, one of the critical amino acids that the system coverts carbon dioxide into. (Image Credit: NASA)
Amino acids are essential for nearly every process in the human body. Often referred to as ‘the building blocks of life,’ they are also critical for commercial use in products ranging from pharmaceuticals and dietary supplements, to cosmetics, animal feed, and industrial chemicals.
And while our bodies naturally make amino acids, manufacturing them for commercial use can be costly — and that process often emits greenhouse gasses like carbon dioxide (CO2).
In a landmark study, a team of researchers has created a first-of-its kind methodology for synthesizing amino acids that uses more carbon than it emits. The research also makes strides toward making the system cost-effective and scalable for commercial use.
“To our knowledge, it’s the first time anyone has synthesized amino acids in a carbon-negative way using this type of biocatalyst,” says lead corresponding author Pamela Peralta-Yahya, who emphasizes that the system provides a win-win for industry and environment. “Carbon dioxide is readily available, so it is a low-cost feedstock — and the system has the added bonus of removing a powerful greenhouse gas from the atmosphere, making the synthesis of amino acids environmentally friendly, too.”
The study, “Carbon Negative Synthesis of Amino Acids Using a Cell-Free-Based Biocatalyst,” published today in ACS Synthetic Biology, is publicly available. The research was led by Georgia Tech in collaboration with the University of Washington, Pacific Northwest National Laboratory, and the University of Minnesota.
The Georgia Tech research contingent includes Peralta-Yahya, a professor with joint appointments in the School of Chemistry and Biochemistry and School of Chemical and Biomolecular Engineering (ChBE); first author Shaafique Chowdhury, a Ph.D. student in ChBE; Ray Westenberg, a Ph.D student in Bioengineering; and Georgia Tech alum Kimberly Wennerholm (B.S. ChBE ’23).
Costly chemicals
There are two key challenges to synthesizing amino acids on a large scale: the cost of materials, and the speed at which the system can generate amino acids.
While many living systems like cyanobacteria can synthesize amino acids from CO2, the rate at which they do it is too slow to be harnessed for industrial applications, and these systems can only synthesize a limited number of chemicals.
Currently, most commercial amino acids are made using bioengineered microbes. “These specially designed organisms convert sugar or plant biomass into fuel and chemicals,” explains first author Chowdhury, “but valuable food resources are consumed if sugar is used as the feedstock — and pre-processing plant biomass is costly.” These processes also release CO2 as a byproduct.
Chowdhury says the team was curious “if we could develop a commercially viable system that could use carbon dioxide as a feedstock. We wanted to build a system that could quickly and efficiently convert CO2 into critical amino acids, like glycine and serine.”
The team was particularly interested in what could be accomplished by a ‘cell-free’ system that leveraged some process of a cellular system — but didn’t actually involve living cells, Peralta-Yahya says, adding that systems using living cells need to use part of their CO2 to fuel their own metabolic processes, including cell growth, and have not yet produced sufficient quantities of amino acids.
“Part of what makes a cell-free system so efficient,” Westenberg explains, “is that it can use cellular enzymes without needing the cells themselves. By generating the enzymes and combining them in the lab, the system can directly convert carbon dioxide into the desired chemicals. Because there are no cells involved, it doesn’t need to use the carbon to support cell growth — which vastly increases the amount of amino acids the system can produce.”
A novel solution
While scientists have used cell-free systems before, one of the necessary chemicals, the cell lysate biocatalyst, is extremely costly. For a cell-free system to be economically viable at scale, the team needed to limit the amount of cell lysate the system needed.
After creating the ten enzymes necessary for the reaction, the team attempted to dilute the biocatalyst using a technique called ‘volumetric expansion.’ “We found that the biocatalyst we used was active even after being diluted 200-fold,” Peralta-Yahya explains. “This allows us to use significantly less of this high-cost material — while simultaneously increasing feedstock loading and amino acid output.”
It’s a novel application of a cell-free system, and one with the potential to transform both how amino acids are produced, and the industry’s impact on our changing climate.
“This research provides a pathway for making this method cost-effective and scalable,” Peralta-Yahya says. “This system might one day be used to make chemicals ranging from aromatics and terpenes, to alcohols and polymers, and all in a way that not only reduces our carbon footprint, but improves it.”
Funding: Advanced Research Project Agency-Energy (ARPA-E), U.S. Department of Energy and the U.S. Department of Energy, Office of Science, Biological and Environmental Research Program.
DOI: 10.1021/acssynbio.4c00359

Professor Pamela Peralta-Yahya, lead corresponding author of the study.

Ph.D. Student Shaafique Chowdhury, first author of the study.

Ph.D. Student Ray Westerberg
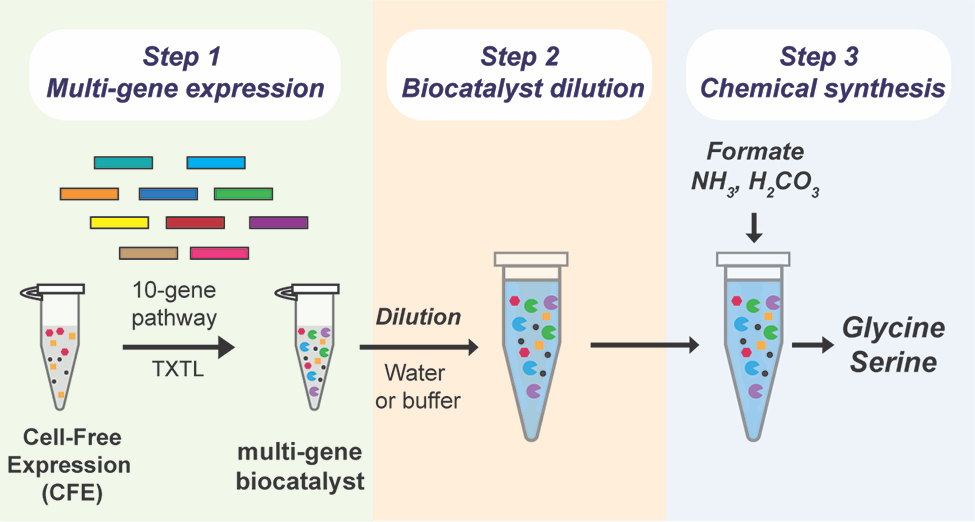
“Part of what makes a cell-free system so efficient,” Westenberg says, “is that it can use cellular enzymes without needing the cells themselves. By generating the enzymes and combining them in the lab, the system can directly convert carbon dioxide into the desired chemicals.”
Written by Selena Langner





