Modeling Bacteria
May 05, 2016 — Atlanta, GA
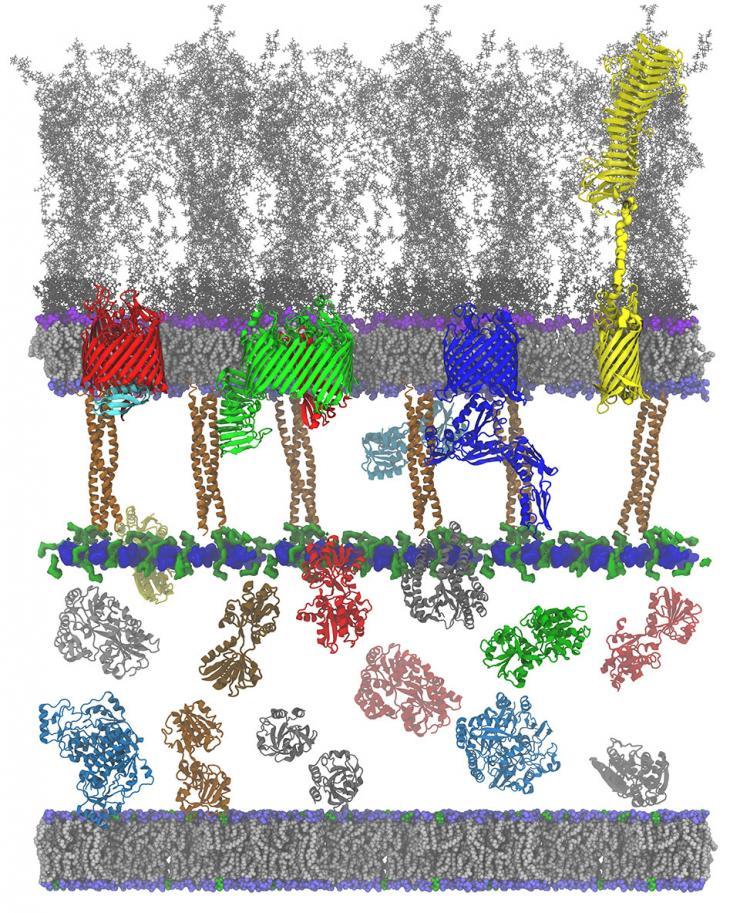
This detailed model shows how proteins interact with the cell membranes of gram-negative bacteria such as E. coli, N. gonorrhoeae and Salmonella. The goal is to find new ways to attack these microorganisms with antibiotics compounds.
Image: Curtis Balusek, Hyea Hwang, James C. Gumbart
Antibiotic resistance in bacteria is among the most critical public health threats today. As more existing antibiotics lose their ability to battle bacteria, there’s pressure to develop new drugs that can attack the bugs in different ways.
Key to that effort is understanding how bacteria operate so new compounds can be developed to attack the microorganisms at their weakest points.
Georgia Tech researchers are modeling gram-negative bacteria such as E. coli, N. gonorrhoeae, and Salmonella to find gaps in their cellular defenses — specifically, their outer cell membranes. Having detailed models of these structures can help experimentalists understand what their research is showing and point to new areas of investigation.
“One of the helpful things about modeling is that often just having a detailed picture of the system shows you what questions you should be asking,” said J.C. Gumbart, a professor in Georgia Tech’s School of Physics. “It’s really important that we have very accurate models to understand how different bacteria interact with the immune system and with potential drugs in diverse ways.”
The bacteria that Gumbart studies are unusual in that they have two outer membranes, one on each side of the cell wall. These membranes are very different from those of other cells, and they have special features that may provide avenues for pharmaceutical attack.
But even the best experiments can’t show all of the factors involved in the membranes’ functions, which is why models can be useful. The models, which run on high-performance computers both locally and at national supercomputing centers such as the one at Oak Ridge National Laboratory, combine experimental data with basic principles of biophysics. “We have effectively infinite resolution, and we can present dynamic, atomistic resolution views of the processes going on there,” Gumbart said.
Georgia Tech researchers are working with colleagues at the National Institutes of Health, Caltech, Emory University, and other institutions to understand bacteria, including the critical protein BamA, which is responsible for protein insertion into the membrane and could therefore be a target for new antibiotics.
— John Toon
Dione Morton




