“Bacterial Litmus Test” Provides Inexpensive Measurement of Micronutrients
Sep 01, 2015 — Atlanta, GA
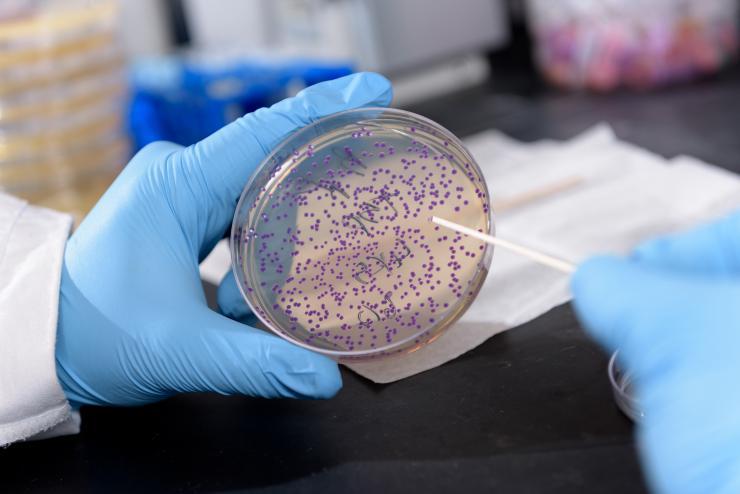
Georgia Tech graduate research assistant Daniel Watstein holds a plate containing E. coli that is producing a purple pigment in response to levels of zinc. The bacterium could be used to detect nutritional deficiencies in resource-limited areas of the world. Purple would indicate low levels of the micronutrient. (Credit: Rob Felt, Georgia Tech)
A bacterium engineered to produce different pigments in response to varying levels of a micronutrient in blood samples could give health officials an inexpensive way to detect nutritional deficiencies in resource-limited areas of the world. This “bacterial litmus test,” which currently measures levels of zinc, would require no electrical equipment and make results visible as simple color changes.
More than a billion people worldwide may be at risk for adequate zinc intake, but measuring zinc levels in blood samples currently requires sophisticated testing equipment not available in many affected areas. If field tests show the biosensor can successfully measure zinc levels, the researchers hope to extend the concept to other micronutrients, including vitamins.
“We think this is just enough technology to meet the needs,” said Mark Styczynski, an assistant professor in the School of Chemical & Biomolecular Engineering at the Georgia Institute of Technology. “The information we can provide could one day help nutritional epidemiologists and non-governmental organizations determine the populations of people that may need interventions to address nutritional deficiencies.”
The proof-of-concept work was reported in the September issue of the journal Metabolic Engineering. The research was supported by the Bill and Melinda Gates Foundation, the National Science Foundation and the National Institutes of Health.
The biosensor is based on modified Escherichia coli (E. coli), a bacterium that is frequently used in genetic engineering. E. coli has a transcriptional system that responds to the level of zinc in its environment, and the researchers have tuned it to trigger the production of purple, red and orange pigments. Genetic machinery for the production of those pigments was taken from other biological sources and introduced into the E. coli.
In practice, health professionals in the field would obtain blood samples from persons suspected of having a zinc deficiency. The blood samples would be spun on a simple mechanical device resembling an eggbeater to separate the plasma from the blood cells. The plasma would then be placed into a test tube or other container with a pellet containing the modified E. coli.
Once mixed with the plasma, the E. coli would multiply, producing the color corresponding to the level of zinc in the blood plasma. Purple would correspond to dangerously low levels, while red would indicate borderline levels, and orange normal levels. The color would be readily visible without any diagnostic or other electronic equipment.
“The process for the color change would take about 24 hours from when the plasma sample is added, though we are hoping to accelerate that,” said Styczynski.
The testing wouldn’t be done to identify individuals in need of treatment, but would be used to assess the nutritional needs of a larger population of people.
“Places where you are likely to encounter micronutrient deficiencies will typically be resource-poor countries, or perhaps locations suffering natural disasters,” Styczynski explained. “These deficiencies aren’t treated on an individual level, but are considered on a population level and used to treat a village or a region that may be affected. We could take samples from 50 or 100 people and be able to assess the nutritional status of an area.”
Because bacteria don’t have the same requirements for many vitamins relevant to human health, the researchers may have to change organisms when they develop tests for other micronutrients, like Vitamin A. Those tests will likely use a yeast organism which has also been extensively studied and into which sensing and pigment-producing genetic machinery can be introduced.
“Ultimately, we hope to be able to test for a whole suite of nutrients in a reasonably short period of time and at a relatively low cost because no equipment would be needed in the field,” Styczynski added.
As part of their research, Styczynski and graduate research assistants Daniel Watstein and Monica McNerney engineered pigment producing machinery into the E. coli. The red and orange colors, lycopene and beta-carotene, are produced by genes taken from Pantoea anantis, a plant pathogen. The purple color, violacein, came from a soil bacterium. Genes for producing the pigments were placed onto a plasmid and introduced into the bacterium.
The researchers used two zinc-sensing proteins within the E. coli and controlled the extent to which those proteins could turn the pigment producing genes on and off. This approach made the zinc-sensing proteins responsive to levels of zinc close to that expected to be found in blood plasma, and can be further used to allow them to turn on at arbitrary levels.
One of the challenges was to avoid producing amounts of pigment that might be toxic to the bacterium, while producing pigment quickly enough to be visible to the naked eye. And because the orange and red pigments are generated in the same metabolic pathway, the researchers needed to establish ways to produce only one or the other at a time – a challenge that their work shows can be feasibly addressed, though they are still working to fine-tune the implementation.
Styczynski believes this system is the first designed to measure blood micronutrients using bacteria without requiring diagnostic equipment. Other techniques have required specialized measurement equipment that is difficult to transport and maintain in the field.
“The general idea of bio-sensing is certainly out there, but we have taken the step of developing a system that doesn’t require equipment in the field,” he said. “We believe this will work well in low-resource areas.”
Among the next steps are development of techniques to freeze-dry the bacterium, and an assessment of the potential ecological impact of the modified bacterium. Styczynski hopes field trials can begin within the next two years.
“This is a convincing proof-of-principle, and we hope to begin the translational aspects of this system based on what we have already shown,” he added. “It’s a matter now of reducing this to practice for something that will ultimately be useful.”
This research was supported by the Bill & Melinda Gates Foundation under grant OPP1046289, the National Science Foundation under grant 1254382, and a National Institutes of Health training grant T32-EB006343. The content of this news release is the responsibility of the authors and does not necessarily represent the official views of the supporting agencies.
CITATION: Daniel M. Watstein, Monica P. McNerney and Mark P. Styczynski, “Precise metabolic engineering of carotenoid biosynthesis in Escherichia coli towards a low-cost biosensor.” (Metabolic Engineering, 2015). http://www.dx.doi.org/10.1016/j.ymben.2015.06.007
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (jtoon@gatech.edu) (404-894-6986)
Writer: John Toon
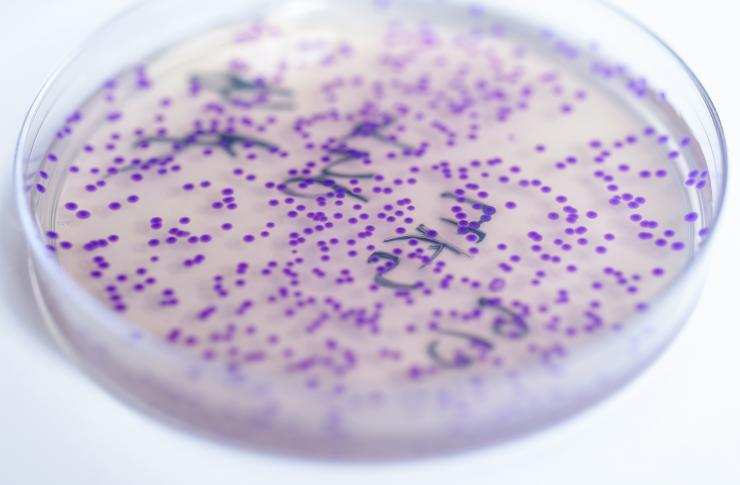
A plate containing E. coli shows a purple pigment in response to levels of zinc. The bacterium could be used to detect nutritional deficiencies in resource-limited areas of the world. Purple would indicate low levels of the micronutrient. (Credit: Rob Felt, Georgia Tech)

Georgia Tech researchers have engineered a bacterium to produce different pigments in response to varying levels of a micronutrient in blood samples. Shown are (left to right) graduate assistant Monica McNerney, assistant professor Mark Styczynski and graduate assistant Daniel Watstein. (Credit: Rob Felt, Georgia Tech)

Georgia Tech graduate research assistant Daniel Watstein holds a plate containing E. coli that is producing a purple pigment in response to levels of zinc. The bacterium could be used to detect nutritional deficiencies in resource-limited areas of the world. Purple would indicate low levels of the micronutrient. (Credit: Rob Felt, Georgia Tech)

Georgia Tech researchers have engineered a bacterium to produce different pigments in response to varying levels of a micronutrient in blood samples. Shown are (left to right) graduate assistant Monica McNerney, assistant professor Mark Styczynski and graduate assistant Daniel Watstein. (Credit: Rob Felt, Georgia Tech)

Georgia Tech graduate research assistant Daniel Watstein and assistant professor Mark Styczynski work in a laboratory on a project to use E. coli bacteria to detect nutritional deficiencies in resource-limited areas of the world. (Credit: Rob Felt, Georgia Tech)




