ATL CleanTech Connect - October 22, 2025
In partnership between Georgia Tech and the Metro Atlanta Chamber, the ATL CleanTech Connect hosts quarterly socials to engage members of the Greater Atlanta clean tech community to support innovation, ideation, startups and investment in clean tech and sustainability focused businesses. Industry, venture capitalists, Georgia Tech faculty and local leaders lead conversations related to cleantech opportunities in the region.
Energy Unplugged: A Georgia Tech Summer Camp Sparks Passion for Energy Innovation Among High Schoolers
Aug 05, 2025 — Atlanta, GA

Rich Simmons and the Energy Unplugged participants during the final day presentations
This summer, the Strategic Energy Institute (SEI) and the Energy Policy and Innovation Center (EPIcenter) hosted Energy Unplugged, an education and outreach program focused on science, technology, engineering, art, and mathematics (STEAM). The annual summer camp is organized through the Center for Education Integrating Science, Mathematics, and Computing (CEISMC), a unit of the College of Lifetime Learning at Georgia Tech. As one of Tech’s most sought-after programs for high school students, the weeklong summer camp continues to spark interest in energy innovation and develop foundational skills in science.
“Energy Unplugged introduces high school students to Georgia Tech’s vibrant innovation ecosystem, engaging young minds in shaping a more forward-thinking energy future,” said Christine Conwell, interim executive director of SEI.
Rich Simmons, SEI’s director of Research and Studies and a George W. Woodruff School of Mechanical Engineering faculty instructor, has led the camp’s curriculum since 2019. Under his leadership, students engage in applied learning experiences that introduce energy efficiency principles, foster creative thinking, and encourage real-world decision-making.
“Energy Unplugged features interactive activities and field trips which provide students tangible exposure to working energy facilities and STEM careers,” Simmons said. “As an integral part of our education and outreach efforts, the camp continues to inspire the next generation to think critically about energy and its impact on their communities and the world.”
“Collaborating with SEI on Energy Unplugged allows us to amplify CEISMC’s mission of expanding access to high-quality STEM experiences,” said Sirocus Barnes, director of Expanded Learning Programs at CEISMC. “By connecting students with real-world energy challenges and Georgia Tech’s research ecosystem, we’re helping them envision themselves as future innovators and problem-solvers.”
The week began with a hands-on workshop where students constructed mousetrap-powered cars, applying core physics concepts and the mechanics of energy conversion. In another activity, students raced remote-controlled cars to highlight the importance of swift decision-making while accounting for external variables. These experiments offered students a dynamic understanding of the relationship between energy and physics. Camp participants also explored electricity use in everyday life by experimenting with solar charging setups, learning how devices like cellphones can be powered through solar energy.
One participant, a rising high school senior, noted the program's differentiation from the typical classroom model: “We had a lot of experiences that aren’t typically offered in high school, which gave me a greater understanding of physics.”
The camp also featured site visits, including a tour of The Kendeda Building for Innovative Sustainable Design — the first building in the Southeast to meet the standards of the Living Building Challenge. Students explored the building’s facilities, including its rooftop garden and photovoltaic canopy. Additional field trips included tours of Oglethorpe’s Georgia System Operations plant and the Morgan Falls hydroelectric power plant, which offered students firsthand exposure to how energy is generated and managed across the state.
To conclude the week, students collaborated in teams on a mini design challenge: devising a sustainable taco business. They were tasked with cooking beans efficiently using either a slow cooker or a pressure cooker and learning how to balance time, energy use, and customer satisfaction. This final project reinforced lessons in energy trade-offs and problem-solving. Teams presented their findings to an audience of parents, faculty, and staff — a memorable opportunity that allowed them to develop public speaking and technical presentation skills as well.
“The presentation on the last day of camp encourages students to use their creativity in different ways to form new solutions and ideas,” said Jake Churchill, graduate student and former camp counselor, “which provides great exposure to an open-minded, nonlinear approach to engineering — and a great teacher, Rich Simmons.”
Contributed by: Katie Strickland

Energy Unplugged camper testing a remote car at Georgia Tech Green

Energy Unplugged camp visit to the Morgan Falls hydroelectric plant
Priya Devarajan || SEI Communications Program Manager
Will People Conserve Energy During Emergency Heat Waves?
Aug 01, 2025 —
This June, New York City’s government and utility urged households to conserve electricity during an extreme heat wave with temperatures reaching 100 degrees F. People were asked to set air conditioners to 76 degrees, to avoid using more than one air conditioning unit, and to delay using electricity-hungry appliances during peak cooling hours.
The big concern is that when every air conditioning unit is running at full blast, electricity demand can exceed total generating capacity and force the utility to implement rolling blackouts. These rolling blackouts avoid a total system failure but leave people without access to cooling and other electronics as temperatures reach dangerous levels.
As temperatures peak in the United States during the coming weeks, utilities and city governments may follow suit with similar requests for voluntary conservation. Voluntary requests for conservation in the United States are part of the standard energy emergency playbook and go back at least to President Carter’s request for Americans to reduce heating temperatures during the 1977 energy crisis.
So, do voluntary conservation requests work to save energy and prevent blackouts?
Written by: Dylan Brewer, Faculty Affiliate, EPIcenter & Assistant Professor, School of Economics, Georgia Tech
Mapping Georgia’s Urban Forest: Georgia Tech Tools Help Planners Prioritize Tree Canopy
Jul 31, 2025 — Atlanta, GA

For more than 15 years, Georgia Tech has provided the City of Atlanta with the foundational data and insight that shape how the city tracks, understands, and plans for changes in its tree canopy. The latest cycle of this research—delivered through the Center for Urban Resilience and Analytics (CURA)—continues that legacy by offering a high-resolution, citywide canopy assessment using satellite imagery and field validation.
The assessment, funded by the city’s Tree Recompense Fund, uses advanced remote sensing tools such as WorldView-2 satellite data and a random forest classification model to categorize land into three land cover types. These include tree canopy, non-tree vegetation (grass, shrubs, and low lying vegetation) and non-vegetation (water, pervious surface). The methodology delivers a detailed spatial picture of land cover across the city.
“This is simply a tool in their planning arsenal,” said Anthony Giarrusso, who has led every canopy study since 2008. “Before they did any of this work in 2008, everything was anecdotal. It was reactionary.”
The new study is not advocacy—it’s information. Giarrusso emphasized that while researchers stay neutral in the politics of urban growth and conservation, their work equips city leaders with science-based knowledge to make more effective zoning and planning decisions.
In addition to mapping existing conditions, the Georgia Tech team developed the Potential Planting Index (PPI), a scalable tool that identifies where tree planting is physically possible based on current land cover. The tool quantifies the difference between tree canopy and non-tree vegetation, indicating zones with restoration potential.
Another key insight is the challenge of interpreting canopy change without understanding land use patterns. “It gives you a false sense of stability if you don’t understand the underlying land use,” said Giarrusso. “You might see canopy regrowth on paper, but that land could be cleared again tomorrow.” He explained that this false signal is particularly common in stalled development sites: “We saw a lot of properties where trees had regrown after initial clearing, but it was temporary and monoculture, low quality canopy. Several of those areas were cleared again for construction later.”
Giarrusso pointed to these “loss-gain-loss” cycles as one of the more misleading aspects of tree canopy analysis without strong land use context. “Some of them were pipe farms—land cleared for development with infrastructure like water and sewer lines installed, but then construction never happened. So trees grow back, and you get a canopy gain that doesn’t last and is nowhere near the quality of the trees originally cleared.”
He stressed that policymakers need to consider the permanence of canopy when using the data. “If it’s just going to be cleared again in two years, it’s not really a gain. That’s why long-term tracking and land use analysis together are so important.”
The city has incorporated these tools into broader planning efforts, including zoning reform and tree ordinance revisions. The research supports recommendations such as restricting full lot clearing in certain zoning categories and adjusting setback or lot coverage limits to better preserve existing canopy.
Giarrusso underscored the urgency of protecting larger, intact forested tracts. “If you can see it from space and it’s still forest—save it,” he said. “Once it’s cleared, you don’t get it back.”
Meet Tommer Ender: Interim Director of the Georgia Tech Research Institute (GTRI)
Jul 30, 2025 — Atlanta, GA

GTRI's interim Director Tommer Ender takes the helm as the organization reaches a new milestone in awards and revenue. During fiscal year 2025, GTRI secured $964 million in new awards, up 11% from the previous year, and earned $980 million in revenue.
Tommer Ender, Ph.D., serves as the interim Director of the Georgia Tech Research Institute (GTRI) and Senior Vice President for Georgia Tech, stepping into the role following the departure of Jim Hudgens, who became President and CEO of UL Research Institutes in June.
Ender takes the helm at GTRI as it reaches a new milestone in awards and revenue. During fiscal year 2025, GTRI secured $964 million in new awards, up 11% from the previous year, and earned $980 million in revenue. GTRI's renowned researchers combine science, engineering, economics, policy, and technical expertise to support national security, the state of Georgia, and industry.
“Tommer has been a driving force behind GTRI’s growth and evolution, and I’m grateful he’s serving in this interim capacity,” said Tim Lieuwen, Georgia Tech’s executive vice president for research. “His deep roots at Georgia Tech — as an alumnus, researcher, and executive — give him a uniquely steady hand at a pivotal time. He leads with both technical expertise and human insight, a rare combination that will serve GTRI well in the months ahead.”
Ender leads over 3,000 GTRI employees and researchers across a variety of disciplines, including autonomous systems, cybersecurity, electromagnetics, electronic warfare, modeling and simulation, sensors, systems engineering, test and evaluation, and threat systems. As interim Senior Vice President of Georgia Tech, Ender also serves on the President’s Cabinet helping set operational and strategic direction for the Institute and reports to Tim Lieuwen, Georgia Tech’s executive vice president for Research.
With nearly 25 years of experience focused on national security and systems engineering research, Ender most recently served as GTRI’s Deputy Director for Research, leading the Electronics, Optics, and Systems Directorate (EOSD). He managed operations for an 800-person unit with an annual $300 million research portfolio across three research laboratories, and was also a member of the GTRI Executive Council, helping set GTRI strategy and informing critical decisions impacting the organization. Ender was previously the Director of GTRI’s Electronic Systems (ELSYS) Laboratory, which has over 500 personnel across 12 locations in the United States.
Ender’s personal area of research includes development of collaborative, executable Model -Based Systems Engineering (MBSE) tools utilizing multidisciplinary design optimization and trade space analytics applied to complex problems. He has also served as an instructor and course developer for Georgia Tech’s Professional Master’s in Applied Systems Engineering, and has been a member of several doctoral and master’s thesis committees at Georgia Tech and other universities.
“For the past two decades, I have had the privilege to work with GTRI’s renowned team of researchers who deliver innovative solutions to the world’s most complex issues,” said Ender. “I am humbled to have been appointed interim Director of GTRI to support our mission focused on national security, improving the human condition, serving the state of Georgia, and educating future technology leaders.”
Ender has been invited to participate in a number of national committees, including at the National Academies of Science, Engineering and Medicine, offering up his expertise in the areas of systems and digital engineering. He is also a Senior Member of the Institute of Electrical and Electronics Engineers (IEEE), and an active member of the International Council on Systems Engineers (INCOSE), National Defense Industrial Association (NDIA), and Military Operations Research Society (MORS), regularly publishing with those organizations.
A three-time alumnus, Ender earned his bachelor’s, master’s and doctorate degrees in Aerospace Engineering from the Georgia Institute of Technology. He is a certified Project Management Professional (PMP) and Certified Systems Engineering Professional (CSEP).
Georgia Tech will be conducting a national search to identify the permanent director of GTRI, with more details to follow.
For more information, please contact gtri.media@gtri.gatech.edu.
To learn more about GTRI, visit: Georgia Tech Research Institute | GTRI

GTRI's interim Director Tommer Ender takes the helm as the organization reaches a new milestone in awards and revenue. During fiscal year 2025, GTRI secured $964 million in new awards, up 11% from the previous year, and earned $980 million in revenue.
Sustainability Workshop for Labs
🐝 Join fellow lab managers to discuss best practices in lab sustainability. Come for one or both sessions!
SCHEDULE
Powering the Future — Without Breaking the Grid
Jul 25, 2025 — Atlanta, GA

As Georgia positions itself as a hub for digital infrastructure, communities across the state are facing a growing challenge: how to welcome the economic benefits of data centers while managing their significant environmental and infrastructure impacts. These facilities, essential for powering artificial intelligence, cloud computing, and everyday internet use, are also among the most resource-intensive buildings in the modern economy.
While companies like Microsoft and Google have pledged to reach net-zero emissions, experts say more transparency and smarter policy are needed to ensure that data center development aligns with community and environmental priorities. That means ensuring adequate energy infrastructure, investing in renewables, training local workers, and mitigating water and carbon impacts through innovation.
A New Kind of Energy Crunch
The rapid rise of AI is fueling explosive demand for computing power — and in turn, energy.
“The proliferation of AI workloads has significantly increased data center energy requirements,” says Divya Mahajan, assistant professor in the School of Electrical and Computer Engineering. “Large-scale AI training, especially for language models, leads to elevated and sustained power draw, often nearing the thermal and power envelopes of graphics processing units systems.”
This sustained demand is particularly challenging in hot, humid regions like Georgia, where cooling systems must work harder. “Training these models can cause thermal instability that directly affects cooling efficiency and power provisioning,” Mahajan explains. “This amplifies reliance on external cooling infrastructure, increasing water consumption and grid strain.”
Environmental and Economic Pressure
“Each new data center could lead to greenhouse gas emissions equivalent to a small town,” says Marilyn Brown, Regents’ and Brook Byers Professor of Sustainable Systems in the School of Public Policy. “In Georgia, the growth of data centers has already led to plans for new gas plants and the extension of aging coal plants.”
There’s an environmental cost to this growth: electricity and water. A single large data center can consume up to 5 million gallons of water per day.
Rising demand has a price. “It’s simple supply and demand,” says Ahmed Saeed, assistant professor at the School of Computer Science. “As overall power demand increases, if supply doesn’t keep up, costs will rise and the most affected will be lower-income consumers.”
Still, experts are optimistic that policy and technology can help mitigate these impacts.
Innovation May Hold the Key
Despite the challenges, experts see opportunities for innovation. “Technologies like direct-to-chip cooling and liquid cooling are promising,” says Mahajan. “But they’re not yet widespread.”
Saeed notes that some companies are experimenting with radical ideas, like Microsoft’s underwater Project Natick or locating data centers in Nordic countries where ambient air can be used for cooling. These approaches challenge conventional infrastructure norms by placing servers underwater or in remote, cold regions. “These are exciting, but we need scalable solutions that work in places like Georgia,” he emphasizes.
What Communities Should Ask For
As communities compete to attract data centers, experts say they should push for commitments that go beyond job creation.
“Communities should ensure that their power infrastructure can handle the added load without compromising resilience or increasing costs,” Saeed advises. “They should also require that data centers use renewable energy or invest in local clean energy projects.”
Training and hiring local workers is another key benefit communities can demand. “Deployment and maintenance of data centers require skilled workers,” Saeed adds. “Operators should invest in technical training and hire locally.”
Policy Can Make the Difference
Stronger policy frameworks can ensure growth doesn’t come at the expense of Georgia’s most vulnerable communities. “We need more transparency from companies about their energy and water use,” says Brown. “And we need policies that prevent the costs of supporting large consumers from being passed on to residential ratepayers.”
Some states are already taking action. Texas passed a bill to give regulators more control over large power consumers. In Georgia, a bill that would have paused tax breaks for data centers until their community impact was assessed was vetoed — but experts say the conversation is far from over.
“Data centers are here to stay,” says Saeed. “The question is whether we can make them sustainable — before their footprint becomes too large to manage.”
Nuclear Power Isn’t What You Think — and That’s a Good Thing
Jul 25, 2025 — Atlanta, GA

Nuclear fuel pellets are primarily made of uranium dioxide (UO2), a ceramic material. They are typically enriched with uranium-235, an isotope that undergoes nuclear fission to produce energy. (Credit: U.S. Nuclear Regulatory Commission)
“Nuclear” is a loaded, highly charged word. It can conjure images — both real and imagined — of explosive destruction.
Nuclear is also a loaded, highly charged technology. A single fuel pellet the size of a pencil eraser contains as much energy as a metric ton of coal, 150 gallons of oil, or 17,000 cubic feet of natural gas.
The technology’s complex history, along with its vast potential, is why nuclear scientists and engineers often find themselves moonlighting as myth busters. Georgia Tech experts are eager to untangle fact from fiction so nuclear can shine — safely.
“I am really excited about nuclear, but this is a technology that has a lot of myths and misinformation around it,” said Anna Erickson, Woodruff Professor in the George W. Woodruff School of Mechanical Engineering (ME), and leader of the Consortium for Enabling Technologies and Innovation (ETI), which is focused on nuclear technology.
“Concerns about nuclear weapons, accidents, and waste have overshadowed nuclear energy’s potential as a clean, carbon-free technology,” she added.
Here, Georgia Tech researchers share what nuclear is, why it’s important, and why its moment is now.
What Is Nuclear?
“Nuclear, as indicated by its name, is focused on the nucleus within an atom, but also the atom as a whole,” said Steve Biegalski, ME professor and chair of the Nuclear and Radiological Engineering and Medical Physics Program. “From an engineering perspective, we're looking at how we can use the physics of an atom — and the physics of a nucleus — to solve different scientific and societal problems.”
In 1938, German and Austrian scientists discovered that breaking apart an atom’s nucleus creates energy through fission. Many aspects of nuclear science, however, were advanced through the Manhattan Project during World War II, in which the U.S. developed the atomic bombs it later dropped on Hiroshima and Nagasaki, Japan. This historical association has likely played a significant role in shaping the negative perception of nuclear technology.
But nuclear science isn’t only about international power and weapons, Biegalski said. Advances in nuclear science have contributed to life-saving cancer therapies, cutting-edge heart scans, and on-demand X-ray technologies.
Safe levels of radiation are all around us — for example, our imported fruits and vegetables are treated with radiation when they enter the country. Even kitty litter is radioactive — not very, but detectable by modern sensors.
“You might have slightly elevated radioactivity for a short while after you eat a banana in the morning,” Erickson said. “Our bodies have evolved to live with radiation.”
AI Has Entered the Chat
Lately, Erickson has been getting calls from major technology companies with questions about how to power data centers. She isn’t surprised — nuclear energy is widely being discussed as the way to power the AI revolution.
“Today’s energy needs are very different than they were in the past, and consistent, reliable, and independent electricity production is necessary — especially for the technology sector,” Erickson explained.
“At this stage, it’s not a question of whether nuclear energy can meet those demands, but how quickly we can make it a reality,” she added.
One of nuclear’s most distinguishing features is its power density, or how much power is produced by volume of raw material. Another defining feature is its reliability. Wind and solar are weather-dependent and provide power intermittently. Nuclear can supply power around the clock, and data centers require that level of consistency.
“There are discussions about developing a number of data centers just outside of Atlanta, and those will require full-size nuclear power plants to power them,” Biegalski said. “When we look at electricity production, these facilities need power 24/7, 365 days a year. Nuclear power can supply that, and wind and solar simply cannot.”
Great Power, Great Responsibility
According to Erickson, the nuclear reactors in use today are far more advanced than those associated with past disasters like Chernobyl and Three Mile Island.
New nuclear plants are designed with great efficiency in mind. Coal must be supplied continuously, whereas nuclear can be loaded once and run for years.
In addition to dispelling misinformation, nuclear experts are also knowledgeable about nuclear nonproliferation and nuclear security. Georgia Tech is a leader in these areas. Experts like Erickson, Biegalski, and their Georgia Tech colleagues are regularly tapped to help design new reactors that are popping up across the country.
The Georgia Tech-led nuclear consortium, ETI, assesses how emerging technologies help or hurt nuclear nonproliferation efforts. Nuclear nonproliferation is the global effort to minimize the spread of nuclear weapons, technology, and development.
“One of our main missions is to understand expansion of civilian nuclear power through the lens of nuclear safeguards and nonproliferation,” Erickson said. “Specifically, we want to know how we can best prevent misuse and mishandling of nuclear materials and keep nuclear facilities safe, while also investing in advancing nuclear technology.”
A Shift in Public Opinion
Despite the popular culture — think Homer Simpson’s nuclear plant job handling green slime — the public is also becoming better informed about nuclear power’s relative safety, especially compared with other energy sources.
In early 2025, nearly 6 out of 10 Americans supported increased development of nuclear energy. But why are Americans gradually coming around to the idea?
Erickson may have the answer. “The technology’s potential is catching on across the globe,” she said. “In France, 70% of their electricity comes from nuclear energy.”
For one of her first research projects as a young student, Erickson analyzed what went wrong with the Chernobyl reactors. She understands why people can be wary of nuclear technology.
“Despite the uptick in support for nuclear, people still have concerns we need to answer, rather than just telling people to trust the experts,” Erickson said. “Talking to people is critical in promoting this technology and making sure we keep the public’s trust in this.”

Anna Erickson

Steven Biegalski
Catherine Barzler, Senior Research Writer/Editor
Institute Communications
catherine.barzler@gatech.edu
Engineering the Car of the Future
Jul 17, 2025 — Atlanta, GA

From the humble beginnings of the three-wheeled Benz Patent-Motorwagen in 1886, the automobile has been a continuous story of technological progress. Each era has seen cars push the boundaries of innovation, evolving from early mechanical systems into sophisticated, computer-driven machines.
We’re now in a new generation of automobiles, where roadways are increasingly shared by electric vehicles (EVs) and autonomous vehicles (AVs).
EVs are projected to dominate global car sales by 2030, according to an RMI report. Meanwhile, AVs are gradually entering the mainstream, with 37 percent of new passenger cars expected to be equipped with advanced driver-assistance technologies by 2035, according to McKinsey & Company.
Georgia Tech School of Electrical and Computer Engineering (ECE) researchers are at the forefront of advanced automotive technologies, working on everything from electric engines and computer vision, to modernizing the power grid to support EV charging.
Given current advancements and future possibilities, ECE is helping bring the future car into view, though many surprises and uncertainties remain. Learn what's on the horizon on the ECE Newspage.
Zachary Winiecki (zwiniecki3@gatech.edu)
How the World’s Nuclear Watchdog Monitors Facilities Around the World – and What it Means That Iran Kicked it Out
Jul 20, 2025 — Atlanta, GA

This travel case holds a toolkit containing equipment for inspecting nuclear facilities. Dean Calma/IAEA, CC BY
What happens when a country seeks to develop a peaceful nuclear energy program? Every peaceful program starts with a promise not to build a nuclear weapon. Then, the global community verifies that stated intent via the Treaty on the Non-Proliferation of Nuclear Weapons.
Once a country signs the treaty, the world’s nuclear watchdog, the International Atomic Energy Agency, provides continuous and technical proof that the country’s nuclear program is peaceful.
The IAEA ensures that countries operate their programs within the limits of nonproliferation agreements: low enrichment and no reactor misuse. Part of the agreement allows the IAEA to inspect nuclear-related sites, including unannounced surprise visits.
These are not just log reviews. Inspectors know what should and should not be there. When the IAEA is not on site, cameras, tamper-revealing seals on equipment and real-time radiation monitors are working full-time to gather or verify inside information about the program’s activities.
Safeguards Toolkit
The IAEA safeguards toolkit is designed to detect proliferation activities early. Much of the work is fairly technical. The safeguards toolkit combines physical surveillance, material tracking, data analytics and scientific sampling. Inspectors are chemists, physicists and nuclear engineers. They count spent fuel rods in a cooling pond. They check tamper seals on centrifuges. Often, the inspectors walk miles through hallways and corridors carrying heavy equipment.
That’s how the world learned in April 2021 about Iran pushing uranium enrichment from reactor-fuel-grade to near-weapons-grade levels. IAEA inspectors were able to verify that Iran was feeding uranium into a series of centrifuges designed to enrich the uranium from 5%, used for energy programs, to 60%, which is a step toward the 90% level used in nuclear weapons.
Around the facilities, whether for uranium enrichment or plutonium processing, closed-circuit surveillance cameras monitor for undeclared materials or post-work activities. Seals around the facilities provide evidence that uranium gas cylinders have not been tampered with or that centrifuges operate at the declared levels. Beyond seals, online enrichment monitors allow inspectors to look inside of centrifuges for any changes in the declared enrichment process.
Seals verify whether nuclear equipment or materials have been used between onsite inspections.
When the inspectors are on-site, they collect environmental swipes: samples of nuclear materials on surfaces, in dust or in the air. These can reveal if uranium has been enriched to levels beyond those allowed by the agreement. Or if plutonium, which is not used in nuclear power plants, is being produced in a reactor. Swipes are precise. They can identify enrichment levels from a particle smaller than a speck of dust. But they take time, days or weeks. Inspectors analyze the samples at the IAEA’s laboratories using sophisticated equipment called mass spectrometers.
In addition to physical samples, IAEA inspectors look at the logs of material inventories. They look for diversion of uranium or plutonium from normal process lines, just like accountants trace the flow of finances, except that their verification is supported by the ever-watching online monitors and radiation sensors. They also count items of interest and weigh them for additional verification of the logs.
Beyond accounting for materials, IAEA inspectors verify that the facility matches the declared design. For example, if a country is expanding centrifuge halls to increase its enrichment capabilities, that’s a red flag. Changes to the layout of material processing laboratories near nuclear reactors could be a sign that the program is preparing to produce unauthorized plutonium.
Losing Access
Iran announced on June 28, 2025, that it has ended its cooperation with the IAEA. It removed the monitoring devices, including surveillance cameras, from centrifuge halls. This move followed the news by the IAEA that Iran’s enrichment activities are well outside of allowed levels. Iran now operates sophisticated uranium centrifuges, like models IR-6 and IR-9.
Removing IAEA access means that the international community loses insight into how quickly Iran’s program can accumulate weapon-grade uranium, or how much it has produced. Also lost is information about whether the facility is undergoing changes for proliferation purposes. These processes are difficult to detect with external surveillance, like satellites, alone.
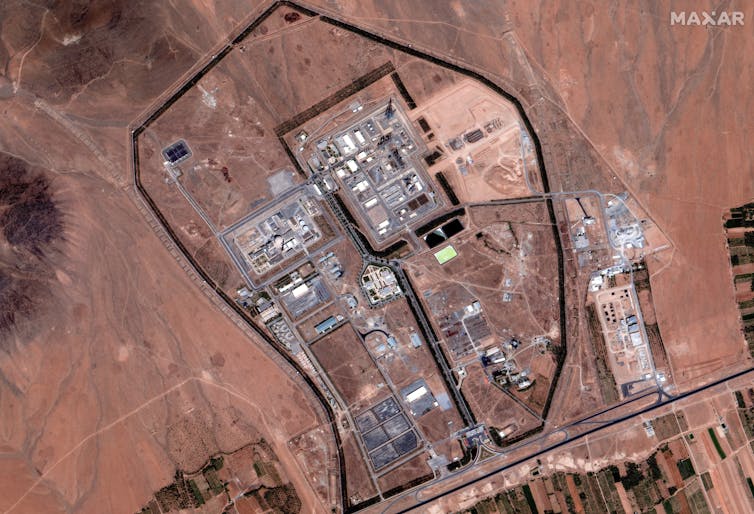
A satellite view of Iran’s Arak Nuclear Complex, which has a reactor capable of producing plutonium. Satellite image (c) 2025 Maxar Technologies via Getty Images
An alternative to the uranium enrichment path for producing nuclear weapons material is plutonium. Plutonium can’t be mined, it has to be produced in a nuclear reactor. Iran built a reactor capable of producing plutonium, the IR-40 Heavy Water Research Reactor at the Arak Nuclear Complex.
Iran modified the Arak reactor under the now-defunct Joint Comprehensive Plan of Action to make plutonium production less likely. During the June 2025 missile attacks, Israel targeted Arak’s facilities with the aim of eliminating the possibility of plutonium production.
With IAEA access suspended, it won’t be possible to see what happens inside the facility. Can the reactor be used for plutonium production? Although a lengthier process than the uranium enrichment path, plutonium provides a parallel path to uranium enrichment for developing nuclear weapons.
Continuity of Knowledge
North Korea expelled IAEA inspectors in 2009. Within a few years, they restarted activities related to uranium enrichment and plutonium production in the Yongbyon reactor. The international community’s information about North Korea’s weapons program now relies solely on external methods: satellite images, radioactive particles like xenon – airborne fingerprints of nuclear activities – and seismic data.
What is lost is the continuity of the knowledge, a chain of verification over time. Once the seals are broken or cameras are removed, that chain is lost, and so is confidence about what is happening at the facilities.
When it comes to IAEA inspections, there is no single tool that paints the whole picture. Surveillance plus sampling plus accounting provide validation and confidence. Losing even one weakens the system in the long term.
The existing safeguards regime is meant to detect violations. The countries that sign the nonproliferation treaty know that they are always watched, and that plays a deterrence role. The inspectors can’t just resume the verification activities after some time if access is lost. Future access won’t necessarily enable inspectors to clarify what happened during the gap.![]()
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Author:
Anna Erickson, professor of Nuclear and Radiological Engineering, Georgia Institute of Technology
Media Contact:
Shelley Wunder-Smith
shelley.wunder-smith@research.gatech.edu
